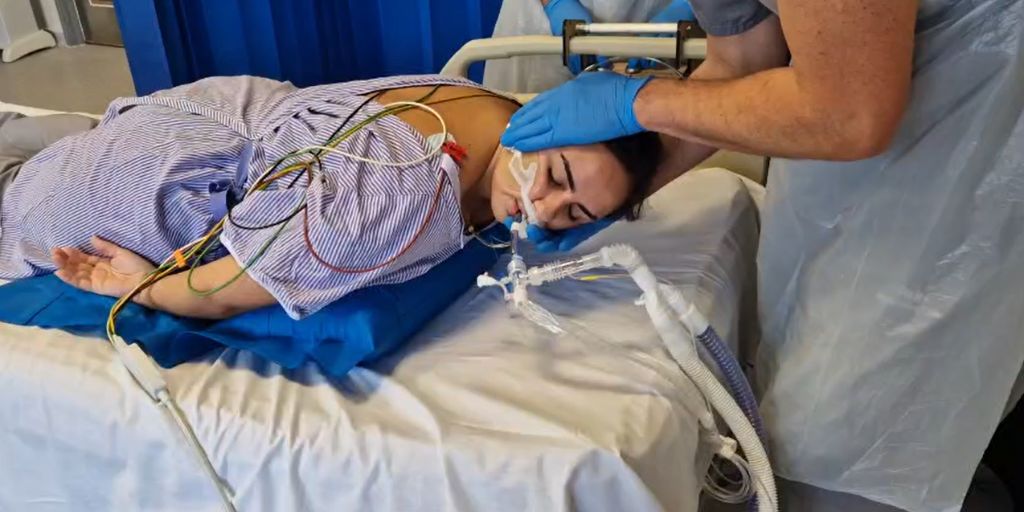
Clinical trials of an innovative inflatable pillow designed to make moving intensive care unit (ICU) patients safer, faster, and less labour-intensive for NHS staff have begun in Bath.
Co-developed by researchers at the University of Bath and clinicians at the Royal United Hospitals Bath NHS Foundation Trust (RUH), and funded by the National Institute for Health and Care Research (NIHR), the Inflatable Prone Repositioning Device – known as the ‘BathMat’ – is a flat balloon-like pillow that can be inflated in sections, and is the first medical device of its kind.
The device was conceived to tackle a major challenge in ICUs: repositioning sedated, ventilated patients in the prone (face-down) position – often among the most critically ill – to avoid pressure injuries and improve oxygenation. Placed under a sedated patient it can gently lift their chest and hips, helping ward staff safely and quickly reposition their head and arms.
The assistance provided by the BathMat has the added benefit of reducing the number of staff needed to move a patient from five to two.
Dr Alexander Lunt, Senior Lecturer in Mechanical Engineering at the University of Bath and the project’s Principal Investigator, said: “Moving critically ill patients is a significant challenge on intensive care wards worldwide. We are pleased to be using our engineering expertise to work toward a better solution to the issue, and to further build our close partnership with our partners at our local NHS Trust, the RUH.”
Trials in four major hospitals
Clinical trials to evaluate the BathMat’s effectiveness began at the RUH in late May. The trials will expand to include Southmead Hospital (North Bristol NHS Trust), Wythenshawe Hospital (Manchester University NHS Foundation Trust), and Derriford Hospital (University Hospitals Plymouth NHS Trust) in the coming weeks.
Aiming to recruit 30 patients across four NHS ICU sites, the trials are backed by a 14-month funding award from the NIHR. Several key outcomes will be measured, including reductions in staff time required for repositioning patients treated lying on their front (prone), improvements in patient healthcare outcomes such as pressure sore prevention, device safety and cost-effectiveness.
Widespread Enthusiasm from Healthcare Staff
Training sessions across the trial sites were met with overwhelmingly positive feedback. Hundreds of ICU staff have now been trained, with many describing the BathMat as a “no brainer” innovation.
Dr Jerome Condry, Chief Investigator and Research Fellow at the Royal United Hospitals Bath NHS Foundation Trust, said: “We’re seeing real enthusiasm from ICU teams who recognise the potential our device has to making repositioning proned patients easier and safer for everyone involved. We aim to have a big impact on both patient safety and team wellbeing.”
Dr Lunt added: “We’ve already had other trusts reach out asking how they can access the device as they see the value it brings immediately.”
Commercialisation and Next Steps
With regulatory approvals in place, the team is now turning its attention to commercialisation. Interest from investors is growing, and efforts are underway to develop a pressure-sensing version of the device that will automatically detect and adjust for pressure hotspots in real time.
Once all trial data is collected and analysed by health economics and statistics experts at the University of Bath, the team will publish the results and begin scaling up to reach more NHS stakeholders and manufacturers. The route to market is being actively developed in partnership with the University’s innovation and research commercialisation teams.
Call for collaborators
The research team welcomes expressions of interest from other NHS trusts or international partners who wish to take part in future trials or demonstrations. Conferences and showcase events are scheduled for 2026 where preliminary results and the next-generation version of the BathMat will be unveiled.
For more information and media resources, visit bathmatmedical.com.











