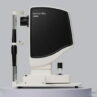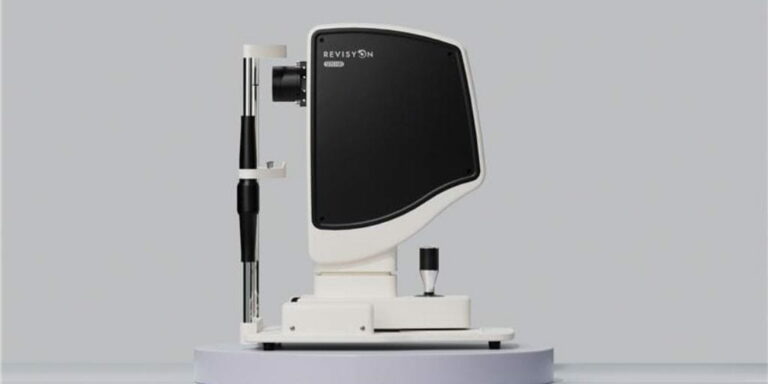
Initial findings from a structured long-term safety and performance follow-up phase of Edinburgh Biosciences’ clinical trial programme for Revisyon™, its non-invasive, LED light-based treatment for people experiencing vision changes associated with cataracts, indicate positive patient feedback.
Completed in January, no adverse events were observed and visual function remained stable post-treatment during the evaluation phase.
Building on clinical trials completed in early 2025, this follow-up study was designed to assess the maintenance of safety and treatment effect, with an average patient follow-up duration of 20 months after the end of treatment.
The longest follow-up period to date extends to 36 months. Detailed clinical data is planned for submission to a peer-reviewed publication in the first half of 2026.
Revisyon™ is a device-led therapy. The treatment applies specific wavelengths of low-level LED light to the crystalline lens using a non-invasive, in-clinic procedure. The technology does not use laser energy and has been developed to work with the eye’s natural structures, with the aim of optimising lens clarity.
Cataracts remain a leading cause of visual impairment worldwide and represent a growing challenge for healthcare systems as populations age. Current care pathways rely on surgery, typically accessed only once visual deterioration has progressed to a significant level, often following prolonged waiting periods.
Revisyon™ has been developed in response to this unmet clinical need and has the ability to transform cataract care, with the objective of enabling earlier intervention within routine eye-care settings. The approach is intended to support people experiencing early vision changes and to complement existing cataract care pathways.
Recognising the advantages of supporting people with cataracts beyond traditional hospital environments, Revisyon has been developed for use in primary care environments, including high street optometry practices, when delivered by trained eye-care professionals. By extending care into non-hospital settings, Revisyon will help alleviate pressure on secondary care services while improving accessibility and convenience for patients.
The technology was initially conceived by Edinburgh Biosciences’ founder, Professor Desmond Smith OBE, based on his extensive research into light-based technologies. This work identified that light of specific wavelengths could interact with lens proteins associated with cataract formation. Following further research and development, the company refined this approach into a clinical application.
Professor Smith passed away in 2023, but his colleagues and family have continued to support the development of the technology in line with his original scientific vision.
Commenting on the completion of the follow-up phase, Alok Machchhar, Chief Commercial Officer at Edinburgh Biosciences, said:
“The completion of the long-term safety and performance evaluation marks a key stage in Revisyon’s development, bringing us a step closer to launch. It has allowed us to better understand how patients experience this treatment; beyond the initial clinical setting.
“The positive early feedback is encouraging and reinforces the importance of exploring alternative treatments for cataract-related vision changes.
“Our focus remains on building a robust clinical evidence base and working responsibly within established care pathways as we prepare for the next stages of development.”
Edinburgh Biosciences expects the full clinical dataset, incorporating trial outcomes and follow-up findings, to be submitted for peer-reviewed publication in the first half of 2026. Any future availability of Revisyon™ will remain subject to regulatory approval.