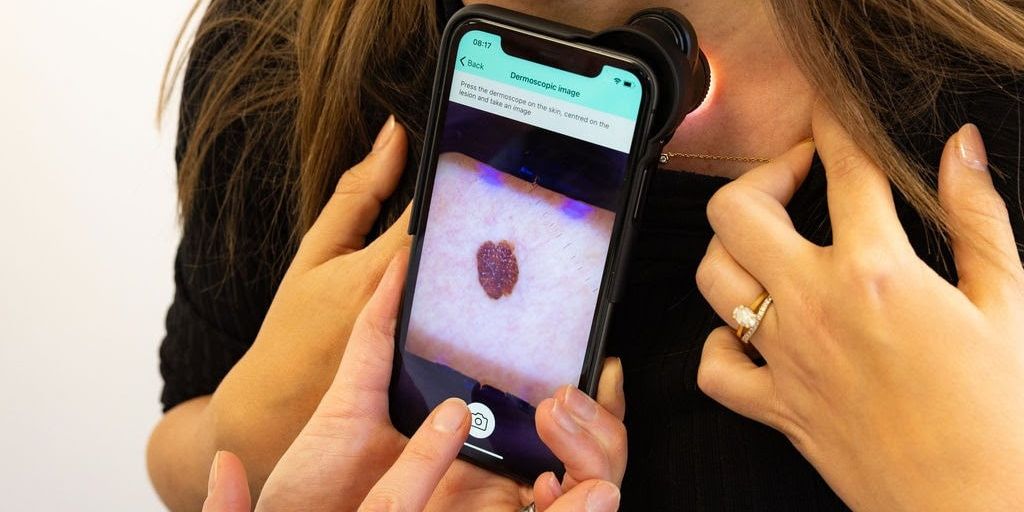
London, UK – [ May 2025]: The National Institute for Health and Care Excellence (NICE) has officially backed a AI tool for detecting skin cancer, marking a major milestone in the use of AI to free up specialist clinician’s capacity and improve outcomes across the NHS. The outcome formally endorses the autonomous use of the technology to discharge patients with benign skin lesions – supporting NHS organisations already using it in this way and providing a clear signal for wider adoption.
The process reviewed a number of technologies and concluded that only the AI medical device DERM, developed by UK healthtech company Skin Analytics should be recommended for use.
DERM is the first AI dermatology solution to achieve two major recognitions: a Class III CE marking under the European Medical Device Regulation (MDR), and a positive recommendation from NICE’s Early Value Assessment (EVA) programme. These significant achievements position Skin Analytics as a global leader in regulated AI, with independent validation of both the safety and effectiveness of its technology.
“The NHS is in crisis. This tool, approved as safe by regulators, has the potential to reassure patients and avoid the necessity for hospital visits. The need to develop new processes to implement safe, effective technologies that will benefit patient care is urgent.”
Dr Julia Schofield, Consultant Dermatologist and former NHS England Clinical Lead for dermatology for the National Outpatient Recovery and Transformation
The NICE approval builds on growing independent scrutiny and recognition of Skin Analytics’ work. In a short space of time, DERM has been featured in an NHS England-commissioned Edge Health report, which analysed how AI could address critical bottlenecks in dermatology. The report highlighted DERM’s ability to safely discharge unnecessary referrals, streamline patient pathways and relieve pressure on specialist services. It was also granted Class III CE marking under the European MDR – the highest level of regulatory clearance for a medical device – and has now been positively evaluated by NICE, paving the way for wider NHS adoption of autonomous AI diagnostics.
As part of this milestone, DERM has received an early-use recommendation from NICE, allowing it to be deployed across the NHS until May 2028 while further evidence is gathered. In practical terms, this means NHS organisations can now adopt DERM using standard NHS funding during a three-year evidence generation period. This enables wider access to the benefits of autonomous AI while building an even stronger case for long-term use, helping address today’s urgent capacity challenges while investing in tomorrow’s smarter, faster care pathways.
This milestone also directly supports the ambitions set out in the NHS 10 Year Cancer Plan, which aims to increase cancer survival rates by diagnosing more cancers earlier and ensuring faster, more equitable access to care. DERM’s ability to triage patients swiftly and safely plays a key role in meeting the goal of diagnosing 75% of cancers at stages one or two by 2028. By reducing delays and expanding diagnostic capacity, autonomous AI tools like DERM help deliver on national priorities of early detection, rapid diagnosis, and improved patient experience, ensuring more people receive timely care, and fewer are lost in the system due to bottlenecks or resource shortages.
Already deployed across 26 NHS sites, spanning both primary and secondary care settings, DERM has been used in pathways that have seen over 165,000 patients and identified more than 15,500 skin cancers. Post-market surveillance shows that DERM detects 97% of cancers, achieving a Negative Predictive Value (NPV) for melanoma of 99.8% – compared to 98.9% for face-to-face dermatologist assessments. In other words, DERM performs on par with, or better than, experienced dermatologists in safely ruling out cancer cases, enabling faster triage, earlier diagnosis, and fewer unnecessary appointments.
Neil Daly, founder and CEO of Skin Analytics, said: “NICE’s endorsement is more than a recommendation. It’s a signal that medical-grade autonomous AI is ready to play a foundational role in modern healthcare. For too long, early cancer detection has been constrained by capacity. With DERM, we now have the opportunity to reimagine how the NHS delivers care: faster, fairer, and at scale. This is just the beginning of what safe, clinically validated AI can do to support patients and overstretched clinicians.”
With over 2.4 million dermatology referrals in 2024, UK dermatology services are under significant pressure. Most urgent referrals do not result in a cancer diagnosis, yet they place enormous strain on capacity and delay access for patients who do need treatment. Routine referrals are also affected, with longer wait times for non-urgent but concerning cases. DERM helps alleviate that pressure by autonomously discharging up to 40% of urgent referrals and helping to reduce face-to-face dermatology appointments by as much as 95% in some settings and freeing up consultant time for those most at risk as a result.
While NICE approval applies specifically to the UK, the organisation is widely respected as a global authority in evidence-based health assessment. This endorsement is expected to influence how other healthcare systems assess and adopt AI diagnostics; setting a benchmark for the safe and scalable use of autonomous AI. It also reinforces the value of clinically validated tools like DERM in helping healthcare systems deliver earlier diagnoses, reduce unnecessary appointments, and make better use of limited clinical capacity.